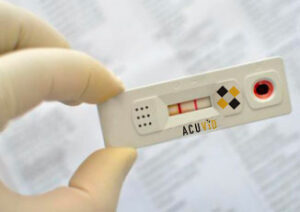
Toronto, Ontario–(Newsfile Corp. – January 20, 2022) – Therma Bright Inc. (TSXV: THRM) (OTCQB: TBRIF) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, announced today that its U.S. Clinical Performance Study is still underway. Once the clinical data has been completed and tabulated, the final results will be reported to the marketplace and the U.S. Food & Drug Administration for Emergence Use Authorization consideration.
“We appreciate the patience of our clients, shareholders, and partners as they await the final results of our U.S Clinical Performance Study,” shared Rob Fia, CEO of Therma Bright. “Our goal is to complete this study in January 2022 within the 300 participants range per our IRB Clinical Trials dashboard. With the rapid spread of the Omicron variant, our clinics and their labs have been overwhelmed with a massive wave of COVID-19 RT PCR testing. This has caused delays in receiving these lab-based tests for several days, thus slowing down the process in matching each participant’s AcuVid™ COVID-19 Rapid Antigen Saliva Test with their specific RT PCR test. Current results are very promising, as we look to be the first COVID-19 rapid antigen saliva test to receive FDA Emergency Use Authorization.”
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.
Source: IRB Clinical Trial Dashboard: https://clinicaltrials.gov/ct2/show/record/NCT05119530?view=record