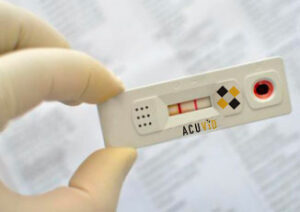
Toronto, Ontario–(Newsfile Corp. – September 14, 2021) – Therma Bright Inc. (TSXV: THRM) (OTCQB: TBRIF) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, announced today its successful up-listing from the OTC Pink Sheets to the OTCQB® Venture Market (the “OTCQB”). Therma Bright will commence trading on the OTCQB with the market open on September 14, 2021, under the symbol “TBRIF”.
“We’re excited to be up-listed to the OTCQB, which is an important milestone for Therma Bright,” shared Rob Fia, CEO of Therma Bright. “The OTCBQ affords us greater visibility within the U.S. investment community, which should enhance our liquidity and increase our access to institutional and retail investors. This additional capital markets exposure will be invaluable as we continue to buildout our AcuVid™, Venowave, Benepod™ and other product lines, along with their U.S. and global distribution sales channels.”
The OTCQB, operated by OTC Markets Group Inc., is designed for developing and entrepreneurial companies in the United States and abroad. Companies must be current in their financial reporting and undergo an annual verification and management certification process, including meeting a minimum bid price and other financial conditions. With more compliance and quality standards, the OTCQB provides investors improved visibility to enhance trading decisions. The OTCQB is recognized by the United States Securities and Exchange Commission as an established public market providing public information for analysis and value of securities. B. Riley Securities acted as the Company’s OTCQB sponsor. B. Riley Securities, Inc. is a full-service investment bank and subsidiary of B. Riley Financial, Inc., based in Los Angeles with offices across the United States, providing corporate finance, research, sales, and trading services.
As reported on September 1st, 2021, the Company successfully completed and submitted additional research and documentation requested by the FDA. Therma Bright has since received an acknowledgement letter from the FDA for the information submitted for the EUA submission. The information has been formally logged into the FDA system and is under review. Therma Bright will continue to work interactively with the FDA to provide additional information, if necessary, to complete the review.
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.
Source: https://ceo.ca/@newsfile/therma-bright-successfully-up-lists-to-the-otcqb-venture