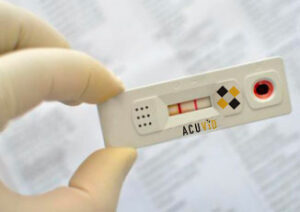
Toronto, Ontario–(Newsfile Corp. – July 22, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to provide an update on its AcuVid™ COVID-19 Rapid Antigen Saliva Test and awaits an official response from the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) review for its COVID-19 rapid antigen test application.
In addition to exceeding the minimum results for FDA-EUA review with the initial 63 tests from the Brazilian AcuVid™ Saliva / RT-PCR Test Clinical Study, which was publicly announced in June 2021, the Company continued to recruit individuals in the clinical study to secure additional real-life patient data on its AcuVid™ saliva test. In total, 264 individuals were enrolled in the study with duration of symptoms that ranged from one (1) day to more than 10 days.
The Company’s overall Brazilian AcuVid™ Saliva / RT-PCR Test Clinical Study, per FDA’s EUA data submission requirements; including PPA (Positive Percent Agreement) and NPA (Negative Percent Agreement), delivered better or comparable results, respectively, to those of Abbott’s BinaxNOW COVID-19 Ag Card Test’s FDA-EUA submission, approved December 2020 (BinaxNOW™ FDA EUA: https://www.fda.gov/media/141570/download).
AcuVid™ COVID-19 Rapid Antigen Saliva Test Results from Brazilian Clinical Study
| Percent Agreement | Percentage | 95% Confidence Interval (CI) |
| PPA (Positive Percent Agreement) | 85.71% | 70.15% – 94.22% |
| NPA (Negative Percent Agreement) | 97.82% | 94.85% – 99.21% |
| OPA (Overall Percent Agreement) | 96.21% | 93.08% – 98.02% |
Note: Positive percent agreement (PPA) is the proportion of comparative/reference method positive results in which the test method result is positive. Negative percent agreement (NPA) is the proportion of comparative/reference method negative results in which the test method result is negative.
“We’re excited to share our overall 264 test results from our Brazilian AcuVid™ Saliva / RT-PCR Test Clinical Study. The additional 201 test results, on top of the initial 63, not only exceed FDA-EUA minimum requirements, but confirms the performance quality and ease-of-use of our AcuVid™ saliva test. In fact, our complete AcuVid™ study achieved comparable, and in some cases, better performance results than other leading COVID-19 rapid antigen tests available on today’s market,” expressed Rob Fia, CEO of Therma Bright. “Our rapid test solution will allow individuals to safely collect their saliva in a saliva collection device and receive accurate positive or negative COVID-19 results within 15 minutes. Best of all, our test beats the uncomfortable experience of having a swab inserted into your nostrils and swirled around to secure a test result.”
Therma Bright’s AcuVid™ COVID-19 Rapid Antigen Saliva Test offers quick results, convenience, comfort and cost affordability; all of which are all key features that customers and global communities want to help stop the spread of this mutating virus. Moreover, the AcuVid™ saliva test has successfully been tested against many of the key variants, including the Delta B.1.617.2, P.1, P.2 and B.1.1.7 variant that continue to devastate our global communities. As many nations prepare for Fall 2021 and the Back-to-School season for pre-K through 12 and college students, top scientists and medical doctors around the world continue to evaluate the impact of COVID-19 and the fast-moving Delta B.1.617.2 variant on those who have or have not been vaccinated. Serial testing will continue to play an important and critical role in mitigating the spread and continuation of this unprecedented global pandemic.
“Our nation, along with so many others, have been battling an unseen enemy for over a year with this ongoing global COVID-19 pandemic'” shared former Pennsylvania Governor Tom Ridge, first Secretary for the US Department of Homeland Security. “Therma Bright’s clinical study including its easy-to-use, 15-minute and smart-enabled COVID-19 antigen saliva test makes it an ideal solution to help our communities deal with the effects of this virus and help to mitigate its spread and variant mutations.”
On March 16, the FDA provided guidance for test developers seeking emergency use authorization (EUA) of certain tests for screening with serial testing. Serial testing involves testing the same individual multiple times within a few days, and can increase chances of detecting asymptomatic infection that might not always show up with a single test. CDC recommends serial testing at least once per week, along with other mitigation measures, such as masking and social distancing, to reduce disease transmission.
The Brazilian AcuVid™ Saliva / RT-PCR Test Clinical Study results are being added to the Company’s applications for ANVISA (Brazil), INVIMA (Colombia) and Health Canada submissions, and will further support the CE approval received in April 2021.
Therma Bright is pleased to announce that it has applied for a Legal Entity Identifier (“LEI”) to maintain its listing on the Frankfurt Stock Exchange. The LEI grants the Company access to European investors, increased liquidity and trading volume and transparency in the global marketplace. The Frankfurt Stock Exchange has regulated that every Issuer of listed securities must have a valid LEI in place to maintain the admission to its markets, thereby following the European Markets in Financial Instruments Regulation. https://www.xetra.com/xetra-en/newsroom/circulars/xetra-circulars/Termination-of-trading-of-securities-of-issuers-without-Legal-Entity-Identifier-LEI–2707646
The Legal Entity Identifier (LEI) is a 20-character, alpha-numeric code based on the ISO 17442 standard developed by the International Organization for Standardization (ISO). It connects to key reference information that enables clear and unique identification of legal entities participating in financial transactions. Each LEI contains information about an entity’s ownership structure and thus answers the questions of ‘who is who’ and ‘who owns whom’. Simply put, the publicly available LEI data pool can be regarded as a global directory, which greatly enhances transparency in the global marketplace.
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.