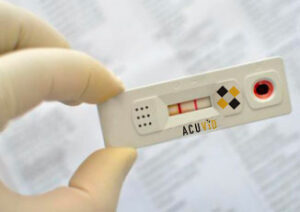
Toronto, Ontario–(Newsfile Corp. – April 22, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce successful preliminary results with its AcuVid™ COVID-19 Rapid Antigen Saliva Test to detect the SARS-CoV-2 Variants of Concern (“VOC”) that are rapidly spreading across Canada and many other countries; specifically the Brazilian P.1 and P.2 and the UK B.1.1.7 variants. The results were obtained in the performance study currently being conducted in Brazil at the Federal University of Minas Gerais (UFMG).
The P.1 SARS-CoV-2 variant was first seen in Manaus, the capital of the state of Amazonas in Brazil. Initial research suggested the virus arose late last year and began spreading in November. It quickly became the dominant strain, leading many in the country to believe it could infect people who had already been infected with the initial strain earlier in the year.
After initially containing COVID-19, many European, Asian and other countries, including Canada, are experiencing a resurgence of COVID-19 in large part because of the evolving SARS-CoV-2 Variants of Concern which are either easier to transmit, cause more severe disease, or both.
To help support global health agencies’ efforts to manage the third wave of this pandemic, Therma Bright initiated and successfully completed a preliminary study on AcuVid™ COVID-19 Rapid Antigen Saliva Test’s ability to detect the Brazilian P.1 and P.2 variants and the UK B1.1.7 variant. Although a relatively small number of samples were used in the initial study, the results showed 100% sensitivity when tested on previously sequenced variant samples.
In the coming weeks, additional studies will be conducted on a larger number of subjects in Brazil under the leadership of Dr. Ricardo Fujiwara in real-life hospital settings to confirm the initial results.
Mr. Rob Fia, CEO of Therma Bright commented, “we are pleased that our AcuVid™ test detects the most important circulating Variants of Concern that are responsible for increased infections and death rates. We feel our test will be able to detect any new variants as they arise. Being able to detect these is crucial, as the number of people being infected with VOCs is rapidly rising worldwide.”
Therma Bright also announces that it has received TSX Venture Exchange (“TSXV”) approval for debt settlements previously announced January 15, 2021. Consequently, the Company has now issued an aggregate of 866,664 common shares which are subject to a hold period expiring August 14, 2021, in accordance with applicable securities laws and the policies of the TSXV.
Therma Bright has negotiated a debt settlement with an arm’s length creditor. Subject to acceptance by the TSXV, the Company has agreed to settle outstanding debt of $100,000 in consideration for which it will issue 200,000 units a deemed price of $0.50 per unit. Each unit is comprised of one common share and one-half of one common share purchase warrant. Each whole warrant will entitle the creditor to purchase one common share for two years from the date of issue at a price of $0.60. All securities issued in relation to this debt settlement are subject to a hold period expiring four months + one day after the date the securities are issued, in accordance with applicable securities laws and the policies of the TSXV.
The Company also announces that it has engaged a consultant to set up a network of manufacturing facilities for US, Canada and European distribution for its anticipated COVID-19 solutions products. Subject to TSXV approval, Therma Bright intends to issue up to 500,000 share purchase warrants to the consultant for the services provided, to be paid in tranches upon certain milestones being met. In addition, The Company has engaged a consultant to provide corporate communications and branding services. Subject to TSXV approval, Therma Bright intends to issue up to 750,000 share purchase warrants to the consultant for the services provided, to be paid in tranches upon certain milestones being met. The warrants listed above will be exercisable for two years and the exercise price will be determined at the time of each issue based on the volume-weighted average closing price of the Company’s common shares on the TSXV for the 5 trading days immediately preceding the date of issue of the warrants.
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.