While we can finally see light at the end of the tunnel in the fight against COVID-19, the global pandemic has invariably changed how we interact with the world around us.
The two big names when it comes to fighting the virus are Pfizer and Moderna, which have been distributed worldwide in various amounts. In addition to companies rushing to make vaccines, antigen test kits are something that innovative companies such as Therma Bright Inc. are striving to produce.
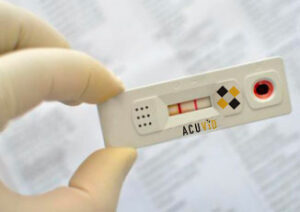
Therma Bright Inc. (TSX-V:THRM, OTC:THRBF, Forum) is a progressive medical device technology company focused on providing quality medical devices that address peoples medical and healthcare needs. Amid the shock and sudden stop to our normal lives, Therma Bright pivoted its business – using its expertise in medical devices – to develop a possible gamechanging technology called AcuVidTM – a rapid, at-home antigen test for detecting the COVID-19 virus in saliva.
In this intriguing investors video podcast, Stockhouse Media’s Dave Jackson was joined by Therma Bright President and CEO, Rob Fia, to discuss the company’s plans to reduce the spread of COVID with the introduction of its gamechanging Acu-Vid test kit, their commercialization strategy for the test kit, and the company pivoted to meet the needs of brought on by the global pandemic.
TRANSCRIPT BELOW:
SH: To start off, how does the AcuVidTM at-home test work? Is it easy for people to use?
RF: Dave, it’s actually very easy to use. It works similar to a pregnancy test. I actually have a sample here. (RF DEMONSTRATES). I’m sure you’ve seen this. It’s a very simple lateral flow test that uses a sponge that you would put under the tongue for 10 seconds to a minute, depending on how much saliva you produce. It has a removable top here, so you can see that transparent piece that would come off. You soak the sponge, then you put that back on it. It turns into a syringe and you would put four drops of saliva into this reservoir, which also acts as a dropper. So you turn that…remove that white cap… you put that dropper on the top, and then what you do is you put four drops into that area there, and in 15 minutes, you get your results. And the way this works, is you’ve got a control line, which has to show up. If it doesn’t show up, the test is faulty. If the control shot a line shows up after 15 minutes and there’s no other line, then you’re negative. If two lines are on this test, then you’re positive for COVID.
SH: Can you explain the difference between Therma Bright’s AcuVidTM test and current test kits in the marketplace…and its competitive advantages?
RF: Well, the competitive advantage we have is the ease of use, the quickness to the result, the costs, we think we’re going to be able to get this down to a price that’s very affordable. We’ll make it scalable so we can produce a lot of these tests. So those are all big advantages. The other tests in the market are PCR tests, which they call the gold standard. People always ask me about this. I mean, the WHO came up with a report that they found the PCR test was measuring very high CT values, and really what you’re detecting there is a dead virus. So you’re detecting the genetic material of the virus, whereas our tests and our initial studies we’ve shown detects CT values at 28 and lower very well. The sensitivity is currently 85% and a hundred percent specific.
We actually noticed that if the individual is showing symptoms within the first seven days, it’s actually a hundred percent sensitive and 100% specific. So that’s very good result for an antigen test like this to be able to get that result quickly. There’s other tests – so you have the nasal pharyngeal, which is the swab that goes deep in the back of the nose. I think it tickles your brain, and then you’ve got the nasal anterior swabs that just go in and around the nostril. Those are the other main competitors in the marketplace. I don’t know of a saliva test. I know there’s saliva tests for PCR and then there’s saliva tests where you spit into a cup, but I’m not too familiar with those. I’ve not seen anything quite like this, where you have a simple swab that’s easy to use for kids or adults or anybody else that wants to do a quick test.
SH: In recent news, the company has entered into Clinical Trial Agreement for its AcuVidTM test kit. Can you walk us through how this entire clinical trial agreement works?
RF: Yeah, so we actually engage with a group here in Ontario. They’re currently running PCR tests. Initially we were going to do the validation in Ontario, and really the validation helps us detect any faults with the tests currently, so that we can improve it when we do the final clinical trial. We actually announced today that we’re doing that test in Brazil. They’re able to turn that around very quickly. So we’ll do the validation in Brazil. We should have those results in two weeks, give or take. And I say give or take because a lot of our shareholders come back and say, ‘Hey, how come this is not done?’ It doesn’t work like that. It’s a very challenging to get researchers and everybody working as quickly as you want them. But we hope to have that in two weeks. Once the validation is done, then we do the 300-person tests, which is used for FDA, CE mark, and with Health Canada. We hope to also potentially do the 300-person test in Brazil, so we have two sites. And then what we need to do is, we’ve applied for IRB approval, the research ethics board approval. We basically pass that. And then what we do from here is we submit to Health Canada for the approval to conduct the test.
SH: What’s the commercialization strategy that Therma Bright’s put together to get this product rolled out to the marketplace and into people’s homes?
RF: That’s a very good question. We kind of looked at that right from the outset because you know, it’s great to have a test like this, but if you can’t scale this up and manufacturer it’s pointless. So we actually have had several conversations with various manufacturers. One in particular, that’s out of the U S has 17 manufacturing locations. They manufacture pregnancy tests, so this is a natural fit for them to manufacture this product. A lot of these items are off the shelf. You can see the molding here, it’s simple injection molding. The strip we know we can do through Nanocomposix, they’re comfortable that they could eventually do something like 25 million strips, which is really the secret in this cartridge (RF holds up cartridge), per month. That’s a lot of strips. This, we get out of a company in the US, (RF holds up swab) and they’re comfortable that by June, they can ramp up to around 25 million swabs a month.
So, you know, we’re starting to put the pieces together to be able to scale. So we’ve got the U.S. group that can help us put it all together, source all the product from these various suppliers. But we also have a Mexican group, that’s also doing pregnancy tests and other medical devices, they’re ready to help us. And we have a group out of Malaysia and then one in Canada. So we’ve thought that through, and then it comes down to selling the product. We’ve had no shortage of individuals and companies and workplace entities, governments, schools, you name it, approaching us to, to sell this product. So I think the demand is definitely there.
SH: Can you tell us about the team behind the Therma Bright name right now. How has management come together to make AcuVidTM possible?
RF: So we actually ran very lean from the start. I’ve been in charge of this company for many years, I was the CEO and we believed in outsourcing everything. We have a lot of other really cool consumer products and all those products are designed outside of this facility, outside of our office. We use engineers, industrial designers. We use manufacturing facilities overseas. So when we’re ready to go, basically we hit a button and away you go, you can manufacture product. So what we did here was, we thought, okay, let’s bring in the experts. I’m no lateral flow expert, but I thought we can bring in some experts, the individual we have on our team, in particular Bruno Maruzzo, actually. Him and I were looking at all of our products. And at the time a lot of our shareholders asked, what can you do something for COVID?
So we went out to look at a facility to manufacture PPE, and we got into a conversation with the fellow that owned it. And he said, well, look, you know, here’s what I do. I’ve been studying SARS and MERS and all these things, and I thought, and he had developed a Rapid TC test for roadside testing. And I said, well, wouldn’t it be great if you could develop a rapid COVID test? And he said, I think we can. So we started working together and that’s how we got into it. So Bruno introduced us to that individual. And then what we did was we brought the team together, We had Roman Zastawny, a lateral flow expert and molecular biologist. Bruno is an electrical engineer, very knowledgeable at making products – he acts more so on medical device companies and boards, we brought in Ammad Shorbajiwho actually used to work at Sanofi and knows the regulatory process.
So we just basically hand-picked our team, and the other key guy is Ian Levine, who has ramped up a lot of small companies that have done $20-25 million in sales upwards of $200-$30 million in sales. And those were all companies in the medical device space, so called him and said, hey Ian if you’re interested, this is a great opportunity if you want to join. So he’s done a great job. Basically, we’ll probably add to the team as we go along. Right now, we’ve got a very good solution to roll this out.
SH: Is there one person on the team that can take credit for the AcuVidTM test, or was it indeed a joint effort?
RF: Well, you know, look, I’d like to (say) we always try to expand our ideas in this company that we’ve done that from the start. And I thought, you know, there’s got to be a solution here for this this massive problem. And we’re always questioning. And I think that’s where, you know, initially my thought to go down this road, kick things off, and then the rest of the team really helped to bring it all together.
SH: With regulating tests such as AcuVidTM, what has been some red tape you’re run into and what has been a concern, if any, from Health Canada or the FDA in the U-S?
RF: Okay. So that’s a bigger challenge than I expected, quite frankly. I mean, I think in Canada it works a lot slower than most other jurisdictions. You know, we’ve got a really great test here, simple tests, we can create jobs in Canada with this product. And there’s a process, there’s this IRB approval, then there’s Health Canada approval just to do the test. And I’ve seen a lot of other companies with antigen tests and antibody tests on the Health Canada website and a lot of them weren’t approved very quickly. I don’t know why, but it is more of a challenge I find doing this in Canada. There’s no doubt about it, but we are going to work through that. I think right now our focus is FDA emergency use authorization. We have written for that, and we’re going to really execute as quickly as we can on the FDA EUA. And then also look for that CE mark as well in Europe. So we may start selling in the US before we sell in Canada, but that also might help with the Health Canada approval because that can, the FDA approval, help with Health Canada.
SH: Rob, pivoting to the investment side of the business, what can you tell our investor audience regarding the current valuation of your stock and why you think it’s a smart buy right now?
RF: Well, listen, I, you know, I’m not going to tell you to buy or sell. But I can say this…if you do the math here, if we can get this test sold for $30, let’s say, and eventually scale that down to say $10 per test. And I can get my costs down to say $2.50 all in costs. You know, there’s some very good margin in there, especially initially. But right now the cost is a little higher because the swab is the most expensive piece. Believe it or not, the rest of this stuff I showed you, the cartridge and the strip, we’re talking pennies here. It doesn’t cost a lot, but the, the swab does cost more. But we’re hopeful that the swab price will come down. So let’s say you make a dollar, a test, you know, and our aim is to do a million tests a month.If we can get to that, and we make a dollar per test, you can do the math. It’s significant.
The demand that we have from the distributors, the workplaces and the governmental buyers that have approached us, far exceeds one-million tests per month. And if we can get to 25 million tests per month, eventually again, you can do the math there, even on a dollar per test, heck of a margin there. So we’re pretty excited about it. I think it’s going to, in terms of valuation currently, the market wants to see what get through the R&D and then they want to know that you can produce it, scale it. And then I think, you know, you could really see a pretty exciting situation there with the stock down the road, in the very near term.
SH: Thanks for taking the time to speak to us today, Rob. Is there anything else you would like to add?
RF: No, Dave, I think that’s it. I think it’s great timing here. Thanks for doing this call. We did put out some news today and I think there’s more news to come. And I think I think all the great shareholders we have and for being patient, and I think that, you know, love to reward all of them and I’m a big shareholder as well. So I want to see this succeed.
Source: Stock House