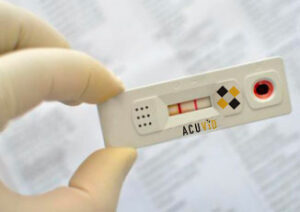
Toronto, Ontario–(Newsfile Corp. – March 18, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), a progressive medical device technology company, is pleased to announce that it has entered into an agreement with Safetest Diagnósticos(“Safetest”) of Brazil in partnership with the Federal University of Minas Gerais to conduct a clinical performance study of its AcuVidTM COVID-19 Rapid Saliva-based Antigen Test.
Brazil’s increase in its COVID-19 infection rate in the past week set another record in terms of new cases and deaths – 12,818 new deaths and more than 464,000 new cases, according to Johns Hopkins University figures. This increase was greater than that seen in the United States, the only country in the world harder hit by the pandemic in absolute numbers.
Because of this, recruitment in this clinical performance study will be completed much faster in Brazil because of the high positivity rate. The study will be conducted under the direction of Dr. Ricardo Fujiwara from the Federal University of Minas Gerais with the support of Safetest. Safetest Diagnósticos was founded in 2016 to bring high quality rapid tests to the market in Brazil and elsewhere. Its first product, the Leishmaniasis Rapid Test, was developed in partnership with the Federal University of Minas Gerais.
Safetest has extensive expertise in the development and validation of antigen and antibody based diagnostic tests with a robust R&D pipeline of diagnostic kits that include tests for Hansen’s Disease, Brucellosis, HTLV and blood screening tests. The study aims to validate a minimum of 60 samples of the AcuVidTM COVID-19 Antigen Test with fresh saliva samples of patients. The samples will be confirmed with an approved PCR test.
Rob Fia, CEO, commented, “The tests will be performed on fresh saliva from patients upon admission to the testing facility. Considering the current positivity in Brazil, we are aiming to complete recruitment and complete the study in less than 2 weeks. The study result will supplement and strengthen the evidence of the clinical value of AcuVid saliva test, an accurate and highly specific, easy to use diagnostic tool to help our fight against this pandemic.”
Mr. Fia went on to say, “Recent lab results of our locked test showed 100% sensitivity and 100% specificity in most patients when tested within 7 days of onset of symptoms. This is the test that will be entering the performance study.”
Dr. Fujiwara commented, “The AcuVID COVID-19 Antigen Test results will be compared to the respective PCR test results which will compare the performance of the tests and will also provide us with real-life data on the ease of use of the device.”
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.