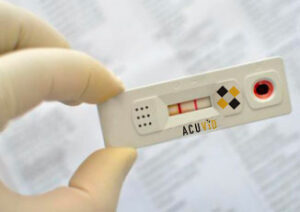
Toronto, Ontario–(Newsfile Corp. – October 4, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), a progressive medical device technology company and developer of the AcuVid™ Covid-19 Rapid Antigen Saliva Test, provides the following update for its AcuVidTM Covid-19 Rapid Saliva Antigen Test.
- Therma Bright filed its application for EUA approval to market its AcuVid™ Covid-19 Rapid Antigen Saliva Test with the US FDA on as previously reported on July 22nd and September 1st, 2021. Therma continues to monitor and interact with the US FDA as necessary regarding our active submission.
- Therma Bright has ordered sufficient materials and components to manufacture the first batch of AcuVid™ tests in the US. At the same time, Therma is securing multiple manufacturing sites in different countries to be able to meet the anticipated demand for our test in the US and elsewhere.
- Therma is identifying and negotiating with potential customers in various jurisdictions to secure initial orders pending regulatory approval.
- In September the Centers for Disease Control and Prevention (CDC) stated that they expect shortages of rapid tests as demand increases. The CDC, and other bodies, recommend serial testing using rapid antigen tests at least once per week, along with other mitigation measures, such as masking and social distancing, to reduce disease transmission, especially in congregant settings such as workplaces, schools and large events.
- Therma is currently identifying sites to perform trials on the AcuVid™ test for home use applications. The results from these trials will be used to file for approval to market the AcuVid™ Covid-19 Rapid Antigen Saliva Test for home use by non-healthcare professionals. This should greatly expand the market for our test. Once a site is identified, Therma expects to report back to shareholders on the expected date to complete the home use clinical study.
Rob Fia, CEO of Therma Bright, commented, “We continue to build manufacturing capacity for our test in anticipation of approval so that we can meet the expected huge demand for rapid testing. Our saliva-based test is easier to use and less intrusive than nasal swab-based tests and is ideally suited for rapid testing for all subjects.”
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.
About Therma Bright Inc.
Therma Bright is a progressive medical device technology company focused on providing consumers and medical professionals with quality medical devices that address their medical and healthcare needs. The Company’s initial breakthrough proprietary technology delivers effective, non-invasive and pain-free skincare. Therma Bright received a Class II medical device status from the US FDA in 1997 for its platform technology that is indicated for the relief of the pain, itch, and inflammation of a variety of insect bites or stings.
Therma Bright Inc. trades on the TSXV: THRM, OTCQB: TBRIF, FSE: JNX. For more information visit: www.thermabright.com and www.coldsores.com