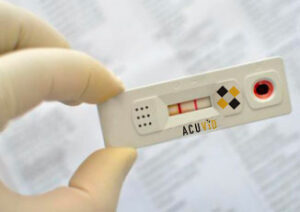
Toronto, Ontario–(Newsfile Corp. – November 12, 2021) – Therma Bright Inc. (TSXV: THRM) (OTCQB: TBRIF) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it will begin its U.S. clinical performance study with the receipt of Institutional Review Board’s (IRB) conditional approval late yesterday, Thursday, November 11, 2021.
The clinical performance study, per U.S. Food & Drug Administration guidance, will being immediately at three (3) U.S.-based clinics. The results will complement the Brazilian clinical study and other requested test data that have been submitted to the FDA over the past four (4) months, beginning in July 2021.
“We’re pleased to have received IRB conditional approval for our U.S. clinical performance study,” shared Rob Fia, CEO of Therma Bright. “Therma Bright can begin moving forward in our efforts with three (3) pre-selected U.S. clinics. Based on current COVID-19 infection rates, we expect the study results to be delivered to the FDA within the next few weeks, as part of our AcuVid™ application for Emergency Use Authorization (EUA).”
Since mid-July 2021, the Company has actively engaged with FDA executives, doctors and scientists on it AcuVid™ COVID-19 Rapid Antigen Saliva Test application, and anticipates Emergency Use Authorization (EUA) following final FDA review.
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.
About Therma Bright Inc.: Therma Bright, developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test, is a progressive medical diagnostic and device technology company focused on providing consumers and medical professionals with quality, innovative solutions that address some of today’s most important medical and healthcare challenges. The Company’s initial breakthrough proprietary technology delivers effective, non-invasive, and pain-free skincare. Therma Bright received a Class II medical device status from the FDA for its platform technology that is indicated for the relief of the pain, itch, and inflammation of a variety of insect bites or stings. The Company received clearance for the above claims from the US FDA in 1997. Therma Bright Inc. trades on the TSXV (TSXV: THRM) (OTCQB: TBRIF) (FSE: JNX). Visit: www.thermabright.com.
Therma Bright Inc.
Rob Fia, CEO
rfia@thermabright.com
Follow us on Twitter
FORWARD-LOOKING STATEMENTS: Certain statements in this news release constitute “forward-looking” statements. These statements relate to future events such as development and commercialization of a rapid COVID-19 viral assay and related instrumentation. as described in the news release. All such statements involve substantial known and unknown risks, uncertainties and other factors which may cause the actual results to vary from those expressed or implied by such forward-looking statements. Forward-looking statements involve significant risks and uncertainties, they should not be read as guarantees of future performance or results, and they will not necessarily be accurate indications of whether or not such results will be achieved. Actual results could differ materially from those anticipated due to several factors and risks. Although the forward-looking statements contained in this news release are based upon what management of the Company believes are reasonable assumptions on the date of this news release, the Company cannot assure investors that actual results will be consistent with these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof and the Company disclaims any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise, except as required under applicable securities regulations.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this press release.
This press release is not an offer of the securities for sale in the United States. The securities have not been registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an exemption from registration. This press release shall not constitute an offer to sell or the solicitation of an offer to buy nor shall there be any sale of the securities in any state in which such offer, solicitation or sale would be unlawful.