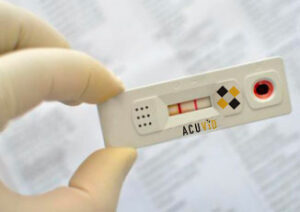
Company advances final validation efforts of its AcuVid(TM) COVID-19 Rapid Antigen Saliva Test
Toronto, Ontario–(Newsfile Corp. – May 27, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to provide the following update on its Brazilian clinical study of its innovative 15- minute COVID-19 antigen saliva test.
Following last week’s announcement on securing K-One MediTech as the Asia-based manufacturer, the Company has finalized the AcuVid™ test kit specifications and is initiating the clinical evaluation study of this simple, easy-to-use and cost-effective kit, and anticipates a full update on the progress from its Brazilian partners at Federal University of Minas Gerais within the next 8 to 12 days.
“This key clinical study requires that each tested participant take our AcuVid™ COVID-19 Rapid Antigen Saliva Test as well as a 24-hour RT-PCR test which will be used as the gold-standard comparator test,” shared Rob Fia, Therma Bright’s CEO. “In order to ensure success in our FDA-EUA (USA), HC (Canada), ANVISA (Brazil) and INVIMA (Colombia) approval and certification, the clinical evaluations for our COVID-19 antigen test must use natural clinical specimens, in order to confirm the performance of our assay. It also requires our test solution to achieve a minimum of 30 positive and 30 negative specimens in the study. We are being vigilant and focused at exceeding these minimum requirements.”
The Company looks to provide a further update upon receipt of the clinical study results. In the meantime, its manufacturing and sales teams continue to prepare for production and distribution of the test.
The Company also reports that it has received TSX-V approval to the debt settlement previously announced April 22, 2021 and has consequently issued 200,000 units at a deemed price of $0.50/unit. Each unit is comprised of one common share and ½ warrant. Each whole warrant entitles the creditor to purchase one common share for two years at a price of $0.60. The Company also advises that it has issued 175,000 warrants to a consultant for services rendered pursuant to a securities for services agreement previously announced April 22, 2021. Each warrant entitles the consultant to purchase one common share for two years at a price of $0.45. All of these securities are subject to a hold period expiring September 27, 2021 in accordance with applicable securities laws and the policies of the TSX-V.
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.
About Therma Bright Inc.
Therma Bright, developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test, is a progressive medical diagnostic and device technology company focused on providing consumers and medical professionals with quality, innovative solutions that address some of today’s most important medical and healthcare challenges. The Company’s initial breakthrough proprietary technology delivers effective, non-invasive and pain-free skincare. Therma Bright received a Class II medical device status from the FDA for its platform technology that is indicated for the relief of the pain, itch, and inflammation of a variety of insect bites or stings. The Company received clearance for the above claims from the US FDA in 1997. Therma Bright Inc. trades on the TSXV (TSXV: THRM) (OTC Pink: THRBF) (FSE: JNX).