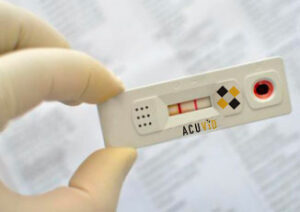
Toronto, Ontario–(Newsfile Corp. – December 16, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to confirm that antibodies incorporated in its AcuVid™ COVID-19 Rapid Antigen Saliva Test have been tested and can successfully detect the new, highly transmissible COVID-19 Omicron B.1.1.529 variant.
Ever since the SARS-CoV-2 Novel Coronavirus (COVID-19) became a global pandemic in early 2020, a variety of new variants have emerged, and the scientific community has worked tirelessly to evaluate each strain and its impact on the public’s health. The Omicron B.1.1.529 variant, discovered in early November 2021 in South Africa and Botswana, has now become a growing global concern, and has been discovered in numerous countries around the world; including the United States, Canada, United Kingdom, and several Western and Eastern European nations.
In fact, on December 3, 2021, the World Health Organization (WHO) confirmed the Omicron variant has been detected in at least 38 countries, and early data suggests it’s more contagious than the Delta B.1.617.2 variant.1The variant “is spreading at a rate we have not seen with any previous variant,” the head of WHO said. “I need to be very clear: Vaccines alone will not get any country out of this crisis.” 2
To date, Therma Bright’s AcuVid™ saliva test solution has successfully detected not only the original Wuhan SARS-CoV-2 Novel Coronavirus (COVID-19), but other WHO Variants of Concern, including the P.1 and P.2 variants discovered in Brazil, the B.1.1.7 variant from the UK, the highly contagious Delta B.1.617.2 variant from India and now the Omicron B.1.1.529 variant from the South Africa region.
“We’re pleased to confirm that the antibodies incorporated in our saliva test have been tested and can successfully detect the Omicron variant,” shared Rob Fia, CEO of Therma Bright. “Now, as our partners finalize our U.S. AcuVid™ clinical performance study to submit to the U.S. Food and Drug Administration for Emergency Use Authorization (EUA), our team is making sure we checkoff each new EUA Guidance; including the September 23, 2021 guidance3 on testing all COVID-19 Variants of Concern, and the November 15, 2021 guidance4 on production capabilities of a minimum of 500,000 tests per week.”
The Company advises that is has issued 100,000 warrants pursuant to a securities for services agreement previously announced on August 12, 2021. Each warrant is exercisable for one common share for two years at a price of $0.39. All of these securities are subject to a hold period expiring April 26, 2021 in accordance with applicable securities laws and the policies of the TSXV.
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.
In other news, Therma Bright has begun fulfilling the first 2500 Venowave 2-unit kit order to DME Authority, the Company’s exclusive U.S. Distributor based out of Nashville, Tennessee. Furthermore, Therma Bright officially launched its Venowave.com ecommerce site for direct-to-consumer sales, as highlighted earlier in its Benepod.com ecommerce site announcement.
Sources:
1. “WHO says Covid omicron variant detected in 38 countries, early data suggests it’s more contagious than delta.” (https://www.cnbc.com/2021/12/03/who-says-omicron-covid-variant-has-spread-to-38-countries.html)
2. Omicron ‘most significant threat’ since pandemic began, U.K. health authority warns (https://www.nbcnews.com/news/world/omicron-significant-threat-pandemic-began-uk-health-authority-warns-rcna8828 )
3.Establishing additional Conditions of Authorization for the EUAs of Certain Molecular, Antigen and Serology IVDs related to viral mutations. (https://www.fda.gov/media/152406/download)
4. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised): Guidance for Developers and Food and Drug Administration Staff. (https://www.fda.gov/media/135659/download )